The Versatile Nature of 4-Dimethylaminopyridine (DMAP) in Organic Synthesis
Related Articles: The Versatile Nature of 4-Dimethylaminopyridine (DMAP) in Organic Synthesis
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The Versatile Nature of 4-Dimethylaminopyridine (DMAP) in Organic Synthesis. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Versatile Nature of 4-Dimethylaminopyridine (DMAP) in Organic Synthesis

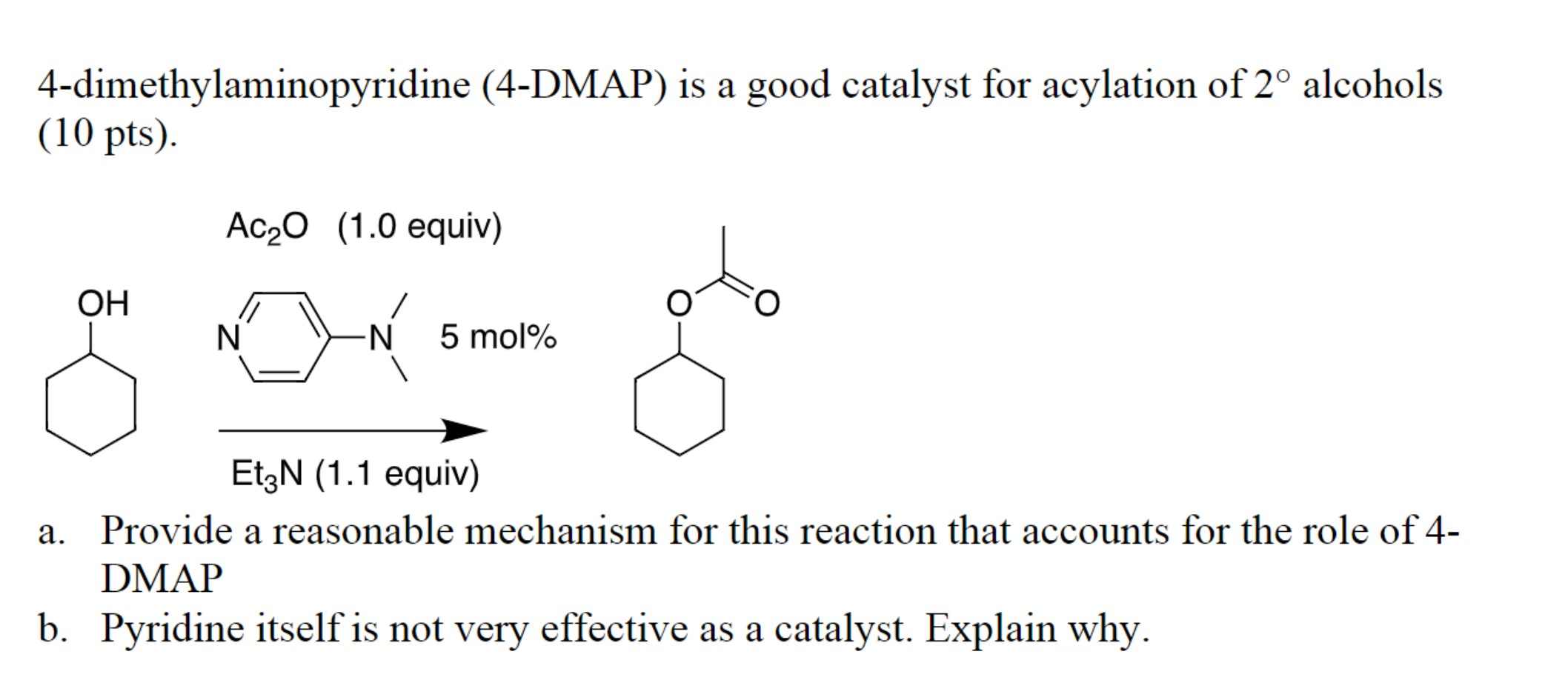
4-Dimethylaminopyridine (DMAP) is a highly versatile and widely used organic compound in chemical synthesis. Its unique structural features and reactivity make it an indispensable tool for a variety of transformations, particularly in the realm of organic chemistry. This article delves into the intricate nature of DMAP, exploring its fundamental properties, diverse applications, and the underlying principles that govern its effectiveness.
Understanding the Structure and Properties of DMAP
DMAP belongs to the family of heterocyclic aromatic compounds, specifically pyridine derivatives. Its structure consists of a six-membered aromatic ring with a nitrogen atom at the 1-position and two methyl groups attached to the nitrogen atom at the 4-position. This seemingly simple structure harbors a remarkable combination of properties that contribute to its significant role in organic synthesis.
Key Properties of DMAP:
- Nucleophilicity: DMAP possesses a highly nucleophilic nitrogen atom due to the electron-donating effect of the two methyl groups. This enhanced nucleophilicity makes it a potent catalyst for a variety of reactions, particularly those involving acyl transfer.
- Basicity: The nitrogen atom in DMAP is also a strong base, allowing it to act as a proton acceptor in various reactions. This basicity further enhances its catalytic activity in certain transformations.
- Steric Hindrance: The presence of the two methyl groups introduces steric hindrance around the nitrogen atom. This steric factor plays a crucial role in controlling the reactivity of DMAP and influencing its selectivity in reactions.
The Role of DMAP in Organic Synthesis
DMAP’s unique combination of properties renders it a powerful catalyst for a wide range of reactions, including:
- Acyl Transfer Reactions: DMAP is renowned for its exceptional catalytic activity in acyl transfer reactions. It efficiently promotes the transfer of acyl groups from acyl halides, anhydrides, or carboxylic acids to nucleophiles such as alcohols, amines, and thiols. This versatility makes it an indispensable tool for the synthesis of esters, amides, and thioesters.
- Esterification Reactions: The ability of DMAP to accelerate acyl transfer reactions makes it an excellent catalyst for esterification reactions. It facilitates the formation of esters from carboxylic acids and alcohols, often under milder conditions compared to traditional methods.
- Amidation Reactions: DMAP is equally effective in promoting amidation reactions, the formation of amides from carboxylic acids and amines. Its catalytic activity in this process allows for the synthesis of a wide range of amides, including those with sterically hindered or sensitive functional groups.
- Ring-Opening Reactions: DMAP can also catalyze ring-opening reactions of cyclic esters (lactones) and cyclic anhydrides. This ability allows for the synthesis of various linear molecules with functional groups at specific positions.
- Nucleophilic Substitution Reactions: In certain instances, DMAP can act as a nucleophile itself, participating in nucleophilic substitution reactions. This property extends its utility beyond catalysis and opens avenues for the synthesis of novel compounds.
Mechanistic Insights into DMAP Catalysis
The catalytic activity of DMAP in various reactions stems from its ability to form highly reactive intermediates. The mechanism typically involves the following steps:
- Activation of the Electrophile: DMAP reacts with the electrophile, such as an acyl halide or anhydride, forming a highly reactive intermediate called an acylammonium salt. This intermediate is stabilized by the electron-donating nature of the dimethylamino group.
- Nucleophilic Attack: The activated electrophile undergoes nucleophilic attack by the nucleophile, leading to the formation of the desired product.
- Regeneration of DMAP: The reaction regenerates DMAP, allowing it to participate in further catalytic cycles.
Applications of DMAP Beyond Organic Synthesis
While DMAP is primarily known for its applications in organic synthesis, its unique properties extend its utility to other fields:
- Polymer Chemistry: DMAP plays a role in the synthesis of polymers, particularly in the preparation of polyesters and polyamides. Its catalytic activity facilitates the formation of these polymers through condensation reactions.
- Materials Science: DMAP finds applications in the synthesis of materials with specific properties. For example, it can be used in the preparation of organic light-emitting diodes (OLEDs) and other electronic materials.
- Analytical Chemistry: DMAP can be used as a reagent in analytical chemistry for the detection and quantification of specific compounds. Its strong nucleophilicity and ability to form stable adducts make it suitable for various analytical techniques.
Safety Considerations and Handling Precautions
DMAP is generally considered a safe compound when handled appropriately. However, it is essential to follow standard laboratory safety practices to minimize potential risks:
- Avoid Contact with Skin and Eyes: DMAP can cause irritation upon contact with skin and eyes. Wear appropriate personal protective equipment, including gloves and safety goggles, when handling DMAP.
- Proper Ventilation: DMAP is a volatile compound, and its vapors can be irritating. Ensure adequate ventilation in the laboratory when working with DMAP.
- Storage: Store DMAP in a cool, dry, and well-ventilated area. Keep it away from sources of ignition and incompatible materials.
FAQs about DMAP
Q1: What is the most common application of DMAP in organic synthesis?
A1: DMAP is most commonly used as a catalyst for acyl transfer reactions, facilitating the formation of esters, amides, and thioesters.
Q2: How does DMAP compare to other catalysts for acyl transfer reactions?
A2: DMAP is often considered a superior catalyst for acyl transfer reactions compared to other commonly used catalysts like pyridine or triethylamine. It exhibits higher reactivity and selectivity, leading to better yields and cleaner reactions.
Q3: Is DMAP a strong base?
A3: Yes, DMAP is a strong base due to the electron-donating effect of the dimethylamino group. Its basicity contributes to its catalytic activity in various reactions.
Q4: What are the limitations of using DMAP as a catalyst?
A4: While DMAP is a highly effective catalyst, it can be sensitive to moisture and air. Its reactivity can also be affected by the presence of strong acids or bases.
Q5: Are there any alternative catalysts to DMAP?
A5: Yes, there are alternative catalysts that can be used for similar reactions, including 4-pyrrolidinopyridine (PPY) and 1-hydroxybenzotriazole (HOBt). However, DMAP often remains the preferred catalyst due to its efficiency and versatility.
Tips for Using DMAP in Organic Synthesis
- Use DMAP in Catalytic Amounts: Typically, only a small amount of DMAP is required to catalyze a reaction, often in the range of 1-10 mol%.
- Optimize Reaction Conditions: The optimal reaction conditions for using DMAP as a catalyst can vary depending on the specific reaction and substrates. Experimentation is often necessary to determine the best conditions for a given transformation.
- Consider the Substrate: The presence of sensitive functional groups or steric hindrance in the substrates can influence the effectiveness of DMAP as a catalyst. Careful consideration of the substrate structure is crucial for successful reaction outcomes.
- Monitor the Reaction: It is essential to monitor the progress of the reaction using appropriate analytical techniques, such as thin-layer chromatography (TLC) or nuclear magnetic resonance (NMR) spectroscopy, to ensure complete conversion and prevent unwanted side reactions.
Conclusion
4-Dimethylaminopyridine (DMAP) is a versatile and powerful reagent in organic synthesis, playing a crucial role in a wide range of transformations. Its unique properties, including its high nucleophilicity, basicity, and steric hindrance, make it an indispensable tool for efficient and selective catalysis. While DMAP is primarily known for its applications in acyl transfer reactions, its utility extends to various other fields, including polymer chemistry, materials science, and analytical chemistry. Understanding the fundamental properties, applications, and mechanisms of DMAP is essential for maximizing its potential and achieving optimal results in organic synthesis.







Closure
Thus, we hope this article has provided valuable insights into The Versatile Nature of 4-Dimethylaminopyridine (DMAP) in Organic Synthesis. We appreciate your attention to our article. See you in our next article!